OneMed Sentinel: Aug 28 2016 Issue
Company Progress Reports -- OneMedResearch
Inspirion Receives FDA Approval for Abuse-Deterrent Opiod
Inspirion Delivery Technologies LLC announced FDA approval of MorphaBond, an oral, extended-release tablet with abuse-deterrent properties, creating the potential for a large-scale reduction in overdoses connected with prescription opiod abuse.
Through various studies and trials, MorphaBond has separated itself as a groundbreaking drug that is resistant to the common forms of manipulation by drug abusers, such as cutting or crushing for intranasal insufflation, injection and smoking.
Nationally, there is a public health crisis with the harmful and life-threatening abuse of prescription pain killers. The misuse and manipulation of prescription opiates can lead to respiratory depression, and death. Its prevalence has led the FDA to meet these challenges by seeking out companies like Inspirion, who seek to curtail the potential for abuse.
MorphaBond is to be administered orally every 12 hours, and will be available in 15, 30, 60 and 100 mg pills.
For more information, visit: http://inspirionrx.com/

NueroRX’s Biopolar Depression Drug Wins Coveted First Place in 2016 ATI-BIOMED Forum Startup Competition
NeuroRX won the coveted first place spot as the most “innovative life science company” at the 2016 Israel Advanced Technology Industry (IATI) BIOMED Forum Conference this past June. Accepting the award of NeuroRX’s behalf was Founder and CEO Dr. Jonathan Javitt.
NeuroRX is a revolutionary clinical-stage pharmaceutical company with disruptive therapeutic solutions. The company was selected as the first prize winner based on its development of Cyclurad, the first oral, fixed-dose drug aimed at treating suicidal symptoms associated with bipolar depression.
Cyclurad sets itself apart from other anti-depressants on the market due to its ability to decrease the risk of suicide in patients taking the drug, by targeting a different chemical receptor in the brain.
The team behind the drug’s development just finished a Phase II clinical trial, with results showing a reduction in symptoms after just two hours of treatment and a sustained benefit lasting more than eight weeks.
The company also recently wrapped up a meeting with the FDA for a crucial study to take place in the third quarter of 2016. NeuroRX is scheduled to complete all clinical trials by close of 2017, along with fast track FDA approval.
Bipolar depression claims over 2,000 lives around the globe each day. There are over five million Americans suffering from the disease, of which approximately 850,000 will eventually commit suicide. Not only are patients with bipolar depression two times more likely to commit suicide as compared with those suffering from other forms of depression, but it is an illness that costs the American healthcare industry hundreds of millions of dollars each year.
NeuroRX’s efforts to address such a life-saving, unmet medical need, particularly in the area of bipolar depression make it a company to watch closely in the coming months.
Learn more at http://www.neurorxpharma.com/

Excision Featured in First Study on Therapeutic Shown to Permanently Inactivate HIV-1
Excision BioTherapeutics was featured in the 2016 issue of Nature Journal, Scientific Reports, under a HIV genome eradication study using CRISPR/Cas9 technology.
The study, conducted by scientists at the Lewis Katz School of Medicine at Temple University, led to the discovery that the HIV virus can be securely and efficiently removed from the DNA of human cells grown in culture.
Excision is an emerging-growth life sciences company, under the guidance of CEO and President Dr. Thomas Malcolm. The company is paving the way in the development of advanced gene-editing medicines to treat HIV, a disease with debilitating symptoms that already led to the deaths of over 25 million and today affects millions in the United States.
There were previously no known studies that successfully eliminated the virus after it had already become integrated into CD4+ T-Cells. Excision’s approach made use of their revolutionary gene editing technology to locate the HIV-1 DNA in the T-cell, and edit the nuclease enzyme. This led not only to eradication of the virus, but perhaps more significantly, a shield against reinfection.
The findings of the study propel Excision to the forefront in the safe treatment and prevention of HIV, and represent a concrete effectiveness of its gene editing system. Excision now plans to move forward with their technology and continue to show itself as a leader in the field.
For more information, visit: http://www.excisionbio.com/

CellAegis Partners with RSK Medical to Distribute Device Aimed at Patients Undergoing Cardiothoracic Surgery
CellAegis inked a deal with RSK Medical Inc. in July 2016 to distribute its innovative autoRIC Device, a non-invasive medical device aimed at improving the outcomes for patients who not only undergo cardiothoracic surgery and interventional cardiothoracic procedures, but also patients with myocardial infarction.
RIC stands for Remote Ischemic Conditioning, and differentiates itself from other devices in that it does not impede other treatments and is user-friendly and easy to administer.
The portable device can be used in a hospital, ambulance or in the home, and has widely been shown to be a safe, convenient and efficient means of delivering therapy to cardiac patients. CellAegis has completed more than 100 trials consisting of more than 20,000 patients around the globe, without a single recorded adverse event.
The partnership with RSK Medical Inc. is significant in that CellAegis joins other cardiology companies known for their disruptive technologies.
CEO Rocky Ganske comes from a strong background in the field of medical devices and diagnostics, having spent over 30 years successfully managing business ventures in similar fields. The company has an equally robust Scientific Advisory Board and management team, all of which contribute to its recent success.
For more information, visit: www.cellaegisdevices.com

Transdermal Announces Addition of Veteran Wallace Reams to Advisory Board
Transdermal Delivery Solutions Corporation (TDSC) announced at the end of August 2016 that Mr. Wallace K. “Wally” Reams will be the newest member of its Board of Advisors.
Kenneth Kirby, President of TDSC, noted that the decision was based on Reams’ stellar reputation and track-record of success in the pharma and medical device fields. Not only does Mr. Reams have over 30 years of experience in transdermal drug delivery, but has also shown a marked success in new business development.
TDSC is an emerging-growth company that focuses on a patented spray-on system of delivering medications directly through the skin. Its technology is unique in that it does not require the use of patches, allows for rapid dosing and can be used for a large, wide-range of pharmaceutical compounds.
TDSC is also involved in the development of a product that treats low testosterone levels in men, called Testagen. The formulation allows for a much quicker absorption versus standard topical testosterone treatments, creating a safe and more efficient product available for the millions of American men who undergo treatment for low testosterone.
For more information, visit: http://www.tdsc.us/

Curtana Pharma Selected in 10 Most Promising Life Science Companies at 2016 Texas Life Science Forum
This past May, the 5th Annual Texas Life Science Forum in Austin honored ten disruptive, emerging-growth companies as the most promising in the field of life sciences.
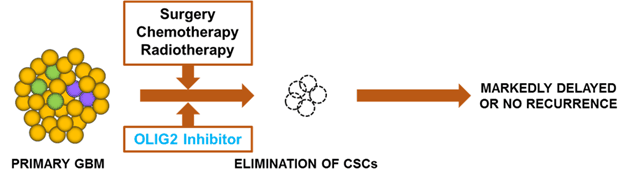
Curtana Pharmaceuticals, a preclinical stage biopharma company, was one of the recipients of this distinctive honor for its work in developing a ground-breaking glioblastoma (GBM) therapy that targets malignant tumors in the brain. It’s three-minute business plan presentation outranked over 50 startup companies who competed.
Leading experts and investors in the field were responsible for assessing each competing company and selecting those which keenly demonstrate an ability to address unmet needs in healthcare.
Curtana’s focus on GBM, widely-considered the most aggressively malignant of a group of tumors called gliomas, allowed the company to target Olig2, which is responsible for the growth of GBM and is unresponsive to chemo and radiation treatments. Curtana has developed a potent inhibitor of OLIG2, disrupting the conventional therapeutic approach and eradicating the potential for tumor growth and reoccurrence.
Brain cancer is a disease that is commonly known as one that offers little treatment options and a window of survival of a little more than a year. However, Curtana, led by CEO Gregory Stein, M.D, M.B.A., and its therapy targeting cancer stem cells in the central nervous system is quickly establishing itself as a state-of-the-art company with the potential to effect life-changing innovations for patients around the globe.
For more information, visit: http://www.curtanapharma.com/